Abstract
BACKGROUND: Clonal hematopoiesis of indeterminate potential/aging (CHIP) is a potential precursor to MDS and myeloid cancer; it is also associated with comorbid cardiometabolic diseases that may be exacerbated by inflammation. The top mutated genes in CHIP are TET2 and DNMT3A (Buscarlet, Blood 2017). Tet2-mutated (deficient) macrophages may contribute to a pro-inflammatory environment (Cull, Exp Hematol 2017), including increased IL-6 (Fuster, Science 2017). Moreover, we found that Tet2-deficient hematopoietic stem cells thrive in an inflammatory environment in vitro, while Tet2 wild-type hematopoietic stem cells are suppressed (Abegunde, ASH 2016).
Thus, we hypothesized that CHIP clones, stratified by mutation type, may be associated with unique comorbid, inflammatory diseases of aging. Specifically, mutated TET2 clones may be more likely to emerge in, or to condition, a pro-inflammatory disease environment (e.g. one containing increased IL-6). Although less is known about the role of DNMT3A in inflammation, we also suspected DNMT3A -mutated CHIP may be associated with similar/unique inflammation and related comorbidity.
METHODS: We analyzed leukocyte genomic DNA from 348 hematologically healthy adults >65 years old (mean age±SD of males and females: 80.6±8.3, 80.2±8.2, respectively) at Baycrest and Sunnybrook Health Sciences Centers (Toronto, Canada). 48 commonly mutated myeloid genes (panel previously applied in Sekeres, JCO 2017) were sequenced using Ion Proton to determine CHIP status (variant allele frequency, VAF>0.02). We collected correlate blood labs and data on 28 comorbidities, enabling the calculation of a Charlson Comorbidity Index and an ECOG performance status score. Data was fitted to logistic regressions to determine odds ratios (OR) for comorbidities relative to CHIP status. Clinical hematological parameters were subject to t-tests. To identify subgroups of CHIP and their respective inflammatory milieus, blood serum cytokine levels were quantified by multiplex assays for a subset of participants (N=298), min-max normalized, and evaluated using Mann-Whitney U tests in SPSS.
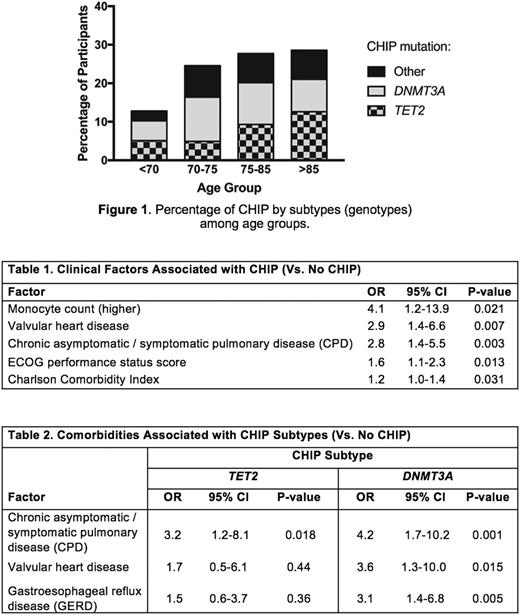
RESULTS: CHIP was detected in 28% of participants overall, increasing with age from 13% (<70 years old) to 29% (>85 years old) (Figure1). The most commonly mutated genes were the epigenetic modifiers TET2 and DNMT3A . CHIP was associated with the presence of valvular heart disease and higher monocyte counts (Table1), supporting previous associations between cardiovascular disease, CHIP and mutant monocytes/macrophages (Jaiswal, NEJM 2014 & 2017). CHIP was also associated with chronic asymptomatic/symptomatic pulmonary disease (CPD), a higher (poor performance) ECOG score, and a higher Charlson Comorbidity Index that translates to reduced predicted 10-year survival.
Investigating subgroups of CHIP compared to people without CHIP, the CHIP group with DNMT3A mutations ("D-CHIP") was more likely to have various comorbid diseases such as gastroesophageal reflux disease, while the TET2 mutations group ("T-CHIP") associated with CPD (Table2). For the first time, we demonstrated that T-CHIP had elevated serum levels of the pro-inflammatory IL-6 (p=0.013) (consistent with murine studies). D-CHIP had elevated serum Eotaxin-1/CCL11 (p=0.028), which is an eosinophil chemo-attractor. Upon removing certain comorbidities as potential confounders, IL-6 and Eotaxin-1 remained elevated in their respective CHIP subgroups. Lastly, those with a CHIP mutation VAF>0.1 had elevated levels of the pro-inflammatory driver TNFα (p=0.012), suggesting a more aggressive inflammatory milieu.
CONCLUSIONS: CHIP is more prevalent than previously reported, possibly due to our aged cohort and the high sensitivity and broad coverage (of TET2 exons) of our method. We report novel connections of comorbidities with CHIP, and find specific comorbidities associating with either or both T-CHIP and D-CHIP subtypes. Moreover, for the first time in humans, we find higher serum levels of certain pro-inflammatory drivers in each CHIP subtype. In the clinical realm, knowing one's CHIP subtype (e.g. T-CHIP) may enable personalized monitoring for specific diseases and preventative, proactive approaches. Ultimately, this may lead to therapeutic avenues that target mutant clones and their specific inflammatory environments to reduce the burden of CHIP and associated comorbidities.
Buckstein: Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.